Learn right there where it all actually happened
Salt. A valuable natural resource that played a huge role in the history of Salzburg.
During your next school trip to Salzwelten Salzburg, we will tell you exactly why that is. Take part at our guided tour through the salt mine, combine theoretical knowledge with hands-on experiences, so that what you learn sticks in your memory even better.
History you can touch and experiment with. We make learning fun!
PACKAGES FOR SCHOOL CLASSES
Salt mine and ...

- Entrance fee and guided tour through the salt mine (duration: ca. 90 minutes)
- Admission to Kelten.Erlebnis.Berg incl. Salt Manufactory
- Entrance and guided school program at Museum of the Celts Hallein (duration: ca. 90 minutes)
For the different themes available to choose from, simply visit the official Museum of the Celts website.
Price: 20,-- EUR per person
(every 11th person free)

- Entrance fee and guided tour through the salt mine (duration: ca. 90 minutes)
- Admission to Kelten.Erlebnis.Berg incl. Salt Manufactory
- Ride up the Zinken on the double chairlift and exciting ride on the Alpine Slide down to the valley (only in good weather, duration ca. 1 hour)
- Discounted admission to the Museum of the Celts and Silent Night Museum Hallein
Price: 25,-- EUR per person
(every 11th person free)
- Entrance fee and guided tour through the salt mine (duration: ca. 90 minutes)
- Admission to Kelten.Erlebnis.Berg incl. Salt Manufactory
- Entrance fee and guided tour of Salzachöfen Gorge incl. zipline
- Discounted admission to the Museum of the Celts and Silent Night Museum Hallein
Price: 28.00 EUR per person
(every 11th person free)

- Entrance fee and guided tour through the salt mine (duration: ca. 90 minutes)
- Admission to Kelten.Erlebnis.Berg incl. Salt Manufactory
- Entrance and guided school program at Museum of the Celts Hallein (duration: ca. 90 minutes)
For the different themes available to choose from, simply visit the official Museum of the Celts website.
Price: 20,-- EUR per person
(every 11th person free)

- Entrance fee and guided tour through the salt mine (duration: ca. 90 minutes)
- Admission to Kelten.Erlebnis.Berg incl. Salt Manufactory
- Ride up the Zinken on the double chairlift and exciting ride on the Alpine Slide down to the valley (only in good weather, duration ca. 1 hour)
- Discounted admission to the Museum of the Celts and Silent Night Museum Hallein
Price: 25,-- EUR per person
(every 11th person free)
- Entrance fee and guided tour through the salt mine (duration: ca. 90 minutes)
- Admission to Kelten.Erlebnis.Berg incl. Salt Manufactory
- Entrance fee and guided tour of Salzachöfen Gorge incl. zipline
- Discounted admission to the Museum of the Celts and Silent Night Museum Hallein
Price: 28.00 EUR per person
(every 11th person free)
For Teachers: Lesson plans
Here you will find not only salt facts, but also experiments. We hope that these facts and hands-on activities will help teachers to enrich their students' experience as they learn about the lifegiving element of salt.
Salt-based Knowledge
Salt is among the earliest and most important natural products, and is crucial to human survival. But not all forms of salt are created equally; the varying forms of salt are differentiated through their extraction methods and origins.
Rock Salt: Mined with Tradition
Rock salt is deeply embedded in the history of Austria and is still mined by hand to this day. Modern tools allow miners to carve large salt stones directly from the salt deposits. These large pieces of salt are later crushed and ground. Only extremely pure veins of salt deposits are suited for this dry mining method.
Among the unique qualities of rock salt from the Salzkammergut region of Austria is how untouched and natural it remains, along with its mild flavor notes and fine light beige coloring. The salt's color can be attributed to the iron content and individual mineral makeup of the region's salt deposits, which contain imporant minerals sich as calcium, magnesium, and iron oxide. No other salt deposit in the world contains the same unique mineral makeup and content.
Other well known sources of unique rock salts include
- the pink Himalayan rock salt of Pakistan
- the Kalahari salt found in the Kalahari desert
- the Persian blue salt of Iran
The varying colors of rock salt reflect the differences in chemical composition and mineral deposits of difference salt sources. In contrast to our native rock salt, other forms of rock salt are imported into Austria.
Rock salt tip: With its natural qualties and mild flavor, rock salt is a great addition to any conscious kitchen.
From Brine to Table Salt
Our BAD ISCHLER Fine Salt (table salt) has its origins in the prehistoric seas that existed 250 million years ago where we now have the Austrian Alps. Salt is a mineral, and consists of sodium and chloride atoms, hence its chemical descriptor of Sodium chloride (NaCl).
Fine salt or table salt is extracted from salt deposits underground as a brine solution that is then evaporated to create the salt crystals we use in our kitchens and at our dining tables.
Through vertically drilled holes, water is pumped into the salt deposits to dissolve the rock salt, creating a cavern through which water is cycled. This begins the process of solution mining. The brine solution created in this mining process is then transported via pipelines to a facility where it is purified and evaporated. The whiter the crystals, the purer the salt.
With a sodium chloride percentage of over 99.9%, BAD ISCHLER Fine Salt is among the purest and highest quality salts - extracted from the Austrian Alps.
Fine salt has far more uses than just seasoning, it is also used in many other ways, including:
- As pharma-salt for medical purposes
- As salt tablets for the purification of drinking water and disinfection of pools
- As industrial and agricultural salt
Fine salt tip: Fine table salt is the ideal salt for fermentation and pickling of foods.
Sea Salt: The Product of Sun and Wind
Sea salt is the created through the evaporation of sea water in natural lagoons or man made drying basins. Through exposure to sun and wind, the water evaporates from the basins, increasing the salinity of the water left behind. As the concentration of salt increases, the water is drained into the harvest basin, where the salt is allowed to crystallize and is scooped out of the water.
Sea water has a salinity of approximately 3.5%, though this percentage varies depending on location. In cooler waters, or brackish waters where fresh water meets the ocean, salt is often at a lower concentration, while higher temperatures create more evaporation and therefore a higher salt content. The Dead Sea, for example, has a salinity of circa 28%.
Typical sea salt is naturally a larger crystal, and contains traces of calcium, magnesium, and manganese.
Sea salt tip: The larger crystals found in sea salt make for a great addition to salt rubs for poultry and other meats.
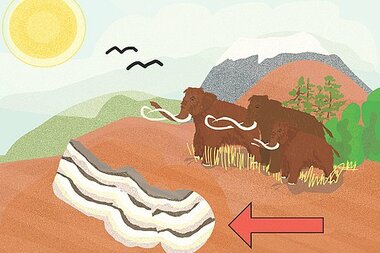
Our Austrian salt deposits were formed around 250 million years ago. But how exactly did this "white gold" end up here in the Alps? The explanation dates back to 1877, with Swedish geologist Carl Ochsenius' "bar theory":

Towards the end of the Paleozoic era, the warming climate led to the evaporation of the lagoon as sea levels fell. Over millions of years, the evaporation process built up the typical deposits of minerals commonly found in sea water: carbonates, sulfates, and salts. The evaporative process continued until the entirity of the sea water in the lagoon had fully evaporated.

In the Mesozoic era, these salt deposits were covered by sand and rubble, then flooded with sea water that formed new salt deposits on top. 240 million years ago, this recurring process of flooding, evaporation, and erosion formed many layers of limestone and dolomite in the region that is now the Salzkammergut.
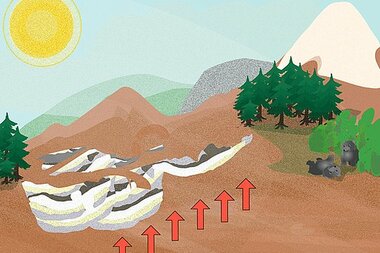
As the Alps began to form approximately 100 million years ago, the salt deposits and the surrounding earth and rock were uplifted, rotated, split apart, and covered with new layers of rock. These relatively malleable salt deposits became encased in other rock, and slowly found their resting place. Through the weight of the limestone mountains, the much softer and more moldable salt was pushed upwards by the pressure, and enveloped in a protective layer of clay. This process is still under way, even as we mine the salt from within the mountain.
Daily salt intake
The body of an adult contains anywhere from 150 to 300 grams of salt, depending on height and weight. Sodium chloride (table salt, chemical formula NaCl) is a crucial mineral for the human body that is required for many necessary functions. Salt is essential in the body, and needed for regulation of blood pressure as well as fluid regulation. A lack of salt, therefore, can negatively impact vital bodily functions.
Osmosis, the basis of cell metabolism also operates based on the salinity of cells and their surrounding environment. Even on a microscopic level, the functioning of the human body relies on salt.
The right dose of salt
So how much salt does the human body need? The World Health Organization (WHO) reccomends that adults consume a maximum of circa 5 grams of salt per day in order to maintain these vital bodily functions. This amount is equal to approximately one teaspoon. Children require less, depending on their age and level of activity.
With a balanced diet, approximately 3/4 of the daily reccomended amount of salt intake will be met. The remaining quarter of the reccomended amount is fulfilled by table salt used for cooking and seasoning. The German Federal Institute for Risk Assessment advises that adults should not consume less than 1.4 grams of salt per day in order to maintain an optimal bodily salt content.
Those who have higher activity levels, such as athletes, should be particularly vigilant about their salt intake. Of course, these needs will vary on an individual basis. so it is best to find a level of salt intake that works for your own body and lifestyle.
Salt-based Experiments
Experiment: Water Dissolves Salt
Materials:
- Glass plate or low bowl
- Salt
- Semolina
- A magnifying glass or microscope
Procedure:
- Add water and some salt crystals to glass container.
- Using the magnifying glass or microscope, observe as the salt crystals shrink in size until they fully dissappear.
- Try the same method with water and semolina. What do you observe?
Experiment: Physical Separation Methods
Materials:
- 1 glass of water
- 1 spoonful of salt
- 1 spoonful of semolina
- Coffee filter
- Cooking pot
Procedure:
- Pour salt and semolina into glass of water, stirring well, until salt has fully dissolved.
- In order to separate this solution of salt, water, and semolina back into its component ingredients, various filtration and seperation methods are required.
- Semolina can be filtered out of the solution by pouring the contents of the glass through a coffee filter; the semolina is visibly separated out and left in the filter. Taste the filtered water. Was the salt filtered out as well?
- In order to separate the salt from the water, a different phyiscal method of separation is necessary. Pour the salt solution into a pot and bring to a boil until the water has evaporated. In the bottom of the pot, a white residue will remain - give it a taste!
Experiment: 3 + 1 = 3 ??
Materials
- Lidded glass jar
- Salt
- Semolina
Procedure
- Fill the glass jar to approximately 3/4 full with water, and mark the level of the water.
- Add your salt, mixing well, until it has fully dissolved. Check the water level after adding and dissolving salt.
- For a comparison, try the same procedure with an equal amount of water and semolina (in the same quantity as the salt in the previous steps). What do you conclude?
Explanation
A salt crystal is composed of many extremely small pieces that are not visible to the naked eye. When a salt crystal comes into contact with water, these little pieces of the salt crystal slowly crumble apart.
In order to understand how this works, it helps to imagine water having little "spaces" or "gaps" between the miniscule components of water. The dissolved salt pieces fit perfectly into these little gaps. Because the salt pieces fit between the water molecules, they seem to dissappear, and the water level remains the same!
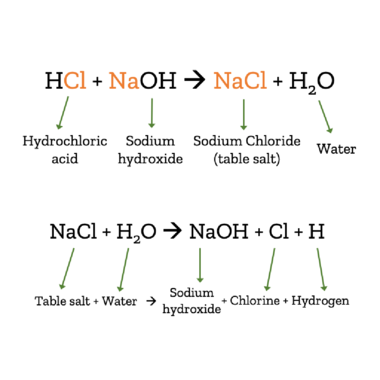
When we talk about salt, we usually mean table salt. In fact, there are many different chemicals that are considered salts. Salts are created when an acid is mixed with a base.
In the case of table salt (chemically: sodium chloride – NaCl), lye/caustic soda (chemically: sodium hydroxide – HCl) combines with hydrocloric acid (HCl). The product of these chemicals when combined is table salt and water. This reaction can also be reversed, turning salt and water back into sodium hydroxide, chlorine, and hydrogen.
Experiment: Separating Table Salt into Sodium and Chlorine
Materials:
- Lidded glass jar
- 2 copper wires
- 3 tsp. salt
- 4.5 volt battery
- Water
Procedure
- Fill jar with water, add 3 tsp. salt and stir until salt is completely dissolved.
- Carefully wrap wires around the two poles of the battery, taking care not to allow them to touch, then lower the wires into the salt solution.
- The solution should now be bubbling at both ends at various levels as the electric current begins to separate the salt into its components of sodium and chlorine.
- The wire on the negative pole of the battery (the longer side) combines the sodium in salt with water molecules to create sodium hydroxide (NaOH). On the positive pole of the battery, yellow-green bubbles, chlorine, are created, most of which binds to the copper in the wire to create copper chloride. If you waft over the jar to carefully smell the reaction, the telltale scent of chlorine should be noticable.
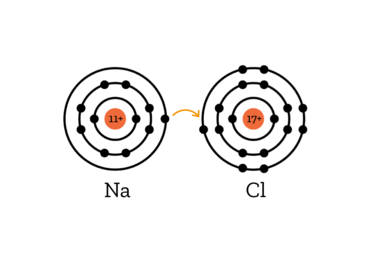
Explanation:
When salt is dissolved in water, the bond between sodium and chlorine is broken. While sodium and chlorin are bonded, the sodiumn atom gives the only electron in its outer shell to the chlorine atom, which then has 8 electrons in its outermost shell, bringing it to a stable noble gas configuration.
When this bond is broken, the sodium atom has one electron too few, creating a positively charged Na+ ion (ions are electrically charged molecules).
The Chlorine, however, has one electron more than usual, creating a negatively charged Cl- ion.
If an electric source is now introduced (for example, our two electrodes placed in the solution), the negative Cl- ions are drawn to the positive pole. They give up their extra electron to return to a neutral electrical charge, and become chlorine gas, which reacts with the copper of the wire and forms copper chloride.
The positive sodium ions (Na+) are attracted to the negative pole, where they gain an electron. In doing so, they become pure sodium metal, which immediately reacts to the surrounding water and becomes sodium hydroxide (NaOH), releasing hydrogen in the process.
Before the invention of canning and refrigeration, one of the most common methods of preserving food was salting. Salt was traditionally used to preserve meats (salting, pickling), fish (eg. salted herring), vegetables (sauerkraut, kimchi), and more. This trick to extend the lifespan of foods dates back as far as Ancient Egypt, where it was used to preserve food for seafaring journeys.
Experiment: Make Your Own Pickles
Materials:
- 1/2 kg small cucumbers
- 1/4 Liter vinegar
- 1/4 Liter water
- 1 tbsp. salt
Procedure:
- Place cucumbers in a bowl and cover with cold water, then let rest overnight. Rinse and dry the next day.
- Add cucumbers and herbs (eg. tarragon, dill, grapeleaves with whole peppercorn) in a clean jar or container in layers.
- Mix your vinegar and water and pour into the container to fully submerge cucumbers.
- Cover the container with a plate, weighed down by a rock or other heavy object.
- Allow the cucumbers to pickle for at least 4 weeks, with regular monitoring. In the case of a layer of yeast or mold forming on the surface, remove and rinse the pickling cucumbers and replace the vinegar solution.
Tip:
To prevent mold and yeast, the pickling solution can be topped with a neutral cooking oil. The oil can later easily be removed from the surface with a spoon.
As far back as 1500 years ago, people in Eastern parts of the globe were already capable of cooling food and drinks. Using crushed ice sprinkled with salt, they created a sort of cooling solution that was able to cool to temperatures as low as -21° celsius.
This method reached Europe hundreds of years later via sea trade. In winter, blocks of ice were cut from frozen lakes and streams to be stored in cellars with straw as insulation in order to save the ice to cool drinks in the summers. When extreme cold was required, the ice could be crushed and mixed with salt to achieve colder refrigeration.
Experiment: Refrigerating Solution
Materials:
- ca. 1/2 kg ice or snow
- 150 g salt
- Glass jar or other container
- Mixing spoon
- Hammer
- Kitchen towel
Procedure:
- Fill kitchen towel with ice cubes and place on a sturdy, shatterproof surface. Using the hammer, crush the ice into small pieces.
- Fill your jar or container with 2-3 cm of crushed ice, topped with a 1 cm thick layer of salt. Repeat this process until full.
- Stir thoroughly, and insert thermometer in the solution before it solidifies to watch the temperature sink.
Explanation:
When salt and water are mixed, the salt will always want to dissolve in the water. In order for this to happen, the water must be liquid, and in the case of this experiment the ice must melt.
The salt begins to melt the surface of the ice, where it immediately dissolves in the resulting water. This reaction requires heat in order to function, and the necessary heat is absorbed from the surrounding environment. As the heat is absorbed by the reaction, the surrounding environment is cooled, reaching temperatures as low as -21° C!
This technique has been used as far back as ancient Roman times. The Romans used salt that built up on the walls of their stables, known as saltpetre or nitrates, primarily Ammonium nitrate. Ammonium nitrate is formed when manure is broken down by bacteria. This technique was also used by Napoleon's armies, though primarily as a byproduct of the production of gunpowder, which consisted of Saltpetre, charcoal, and sulfur.
We often see photos of people floating in the Dead Sea, perhaps even reading a book as they lay suspended in the water. It is one of the few places on Earth where you can float on the water as if lying on an air mattress. This works only becauye of the high concentration of salt in the water.
The water in the Mediterranian Sea has a salinity of ca. 3%. The Dead Sea on the other hand has a salinity of a whopping 30%! Very few organisms can survive such concentrations of salt, which is the root of the name "Dead Sea." Without moving to swim, a human would sink in fresh water. Similarly, a fresh egg will also sink in fresh water.
Experiment: The Suspended Egg
Materials:
- Glass jar or other clear container, full of water
- Tablespoon
- Salt
- 1 fresh egg
Procedure:
- Using your spoon, carfully lay the egg on the bottom of your container.
- Add one tablespoon of salt to the water, and stir gently, careful not to break the egg. What happens?
- Add more salt to the water until the egg floats. The egg is more bouyant in salt water than in fresh water.
- How might we get the egg to sink again?
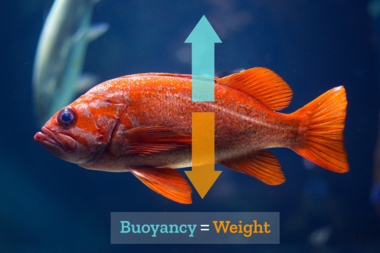
Explanation:
The reason for this phenomenon is bouyancy: every object is lifted in water in direct proportion to the weight of the water it displaces.
A 3 dm³ large stone in air weighs approximately 7.5 kg. In water, however, it weighs 3 kg less, as it displaces 3 dm³ of water (equal to 3 kg). Therfore, the same stone in water only weighs 4.5 kg.
If the weight of the object is:
- greater than its bouyant force (eg. iron - 7.8 kg/dm³) the object will sink.
- less than its bouyant force (eg. wood 0.5 kg/dm³) the object will float.
- equal to its bouyant force, (eg. fish) the object will remain suspended in the water, niether floating to the surface nor sinking.
The egg has a density of approximately 1.1 g/cm³. Fresh water has a density of 1 g/cm³, meaning the egg will sink. The saltwater of the Dead Sea has a density of 1.3 g/cm³, meaning the egg will float to the surface.
Experiment: Growing Salt Crystals
Materials:
- ca. 5 teaspoons fine salt
- Hot water
- 2 heat safe glasses
- A spoon
- A pen or pencil
- A coffee filter or paper towel
- A natural thread (wool or twine)
Procedure:
- Heat the water in a kettle or pot (Kids: ask an adult for help with hot water!).
- Add ca. 4 teaspoons of salt to a glass and carefully pour in ca. 150 ml hot water. Stir until the salt is fully dissolved.
- When the salt has fully dissolved, repeat the process until no more salt will dissolve in the water. This means the solution is saturated, as it will no longer take in the salt, which instead collects at the bottom of the glass.
- Separate the solution from the no longer soluble salt by straining it through the coffee filter or paper towel. Fill the second glass with the solution.
- Wrap the thread around your pen or pencil, then lay it across the top of the glass, taking care that the thread is in contact with the saltwater solution.
- Place your glass in a warm location, such as on or near a radiator. Wait a few days, and with patience, your salt crystals will take shape.
Tip - the longer you wait, the larger the salt crystals will get!
Worth Knowing Before Your Visit
The themes and information of the "Underground Classroom" experience are tailored to children between the ages of 6 and 15 years old.
The guided tour through the salt mine takes approximately 90 minutes. Don't forget to plan in some extra time to visit the Celtic Mountain (Kelten.Erlebnis.Berg) - addmission included in your ticket - directly across from the salt mine's visitor center, where you can spend as much time exploring as you'd like.
In order to provide the best experience possible for our schools and their students, we limit our tour capacity to a maximum of 60 people. If your group will have to be split into multiple tour groups, the tour times will be approximately 30 minutes apart. Our tip to pass the time without missing out on the fun: Explore the Celtic Mountain (Kelten.Erlebnis.Berg)! Admission is included in your tickets, so wander across from the mine entrance and visitor's center to immerse yourselves in life as it was 2,500 years ago on the Dürrnberg, and return to the modern age in no time with the exhibition salt manufactory.
Within the salt mine in Salzburg, we have a year round temperature of approximately 10° Celsius, rain or shine, so prepare for cooler temperatures. Be sure to remind students to come dressed warmly with sturdy footwear for their comfort and safety, so nothing stands in the way of a good time in the mine!
We offer free parking for busses directly adjacent to the entrance of the salt mines.

Reserve now
Make a reservation for your class now: contact us by email at info@salzwelten.at or by phone +43 61 32 200 2400.
Contact